Efficient Synthesis of Nanoscale Transition Metal Borides
- Technology Application
- Method enables the scale-up of transition metal borides at reduced temperatures, time and costs.
- Detailed Technology Description
- None
- Others
-
Background
Transition metal borides are interesting compounds with unique properties. They may be used for various industrial applications such as batteries, superconductors, hard materials, catalysts among other uses. However since they are difficult to synthesize they are not extensively used in commercial applications. Current synthetic methods for these compounds such as solid state metathesis (SSM), arc melting and carbothermal reduction result in reaction products with large particle sizes, uncontrolled crystallization and mixed phase products. Also, the synthesis of transition metal borides requires temperatures above 1,000 °C or requires the use of highly reactive and toxic chemicals. Methods to synthesize these compounds at reduced temperatures and that involve the use of less toxic and reactive starting materials are highly desirable
Related Materials
Tech ID/UC Case
29218/2017-861-0
Related Cases
2017-861-0
- *Abstract
-
Researchers at UCR have developed a simple and efficient transition metal boride synthesis. The transition metal borides are synthesized by directly heating metal chloride and elemental boron in the presence of reducing tin (Sn) between temperatures of 700-900 °C for about eight hours. The resulting transition metal boride products are single-phase nanocrystalline materials with an average size of 100 nm. MoB2, MoB, Mo2B4, Mo2B, CoB, FeB, VB2, NbB, NbB2, TaB2 and WB were all synthesized using this new synthetic method.
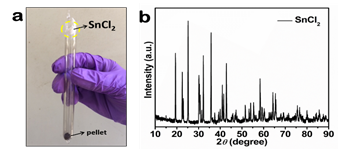
Fig. 1a shows a sealed quartz tube that was heated to ~800 °C. The pellet at the bottom of the tube contains the desired transition metal boride product. The top of the tube contains crystallized SnCl2. Fig. 1b is an X-ray diffraction (XRD) pattern taken of crystallized SnCl2.
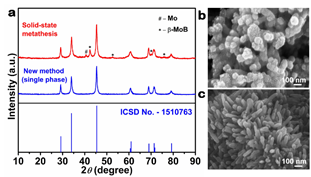
Fig. 2a is a comparison of the XRD patterns of MoB2 synthesized by the previously known method of solid state metathesis (red) and the new method described herein (blue). Fig. 2b is a high resolution scanning electron microscope (HRSEM) image of MoB2 synthesized by previously known solid state metathesis (SSM-MoB2) and Fig. 2c shows materials synthesized by the new method. SSM-MoB2 is contaminated by β-MoB and Mo, whereas Sn-MoB2 reaction products are single phase without contamination. HRSEM shows nanospheres and nanorods for SSM-MoB2 and Sn-MoB2, respectively.
- *Principal Investigator
-
Name: Boniface Fokwa
Department:
Name: Palani Jothi
Department:
- Country/Region
- USA






